We can measure atmospheric pressure, the force exerted by the atmosphere on the earth’s surface, with a barometer (Figure below). A barometer is a glass tube that is closed at one end, filled with a nonvolatile liquid such as mercury, and then inverted and immersed in a container of that liquid. The atmosphere exerts pressure on the liquid outside the tube, the column of liquid exerts pressure inside the tube, and the pressure at the liquid surface is the same inside and outside the tube. The height of the liquid in the tube is therefore proportional to the pressure exerted by the atmosphere.
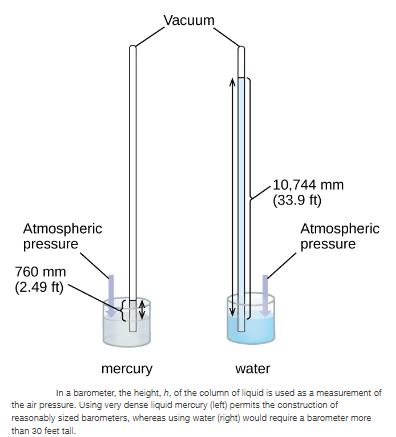
If the liquid is water, normal atmospheric pressure will support a column of water over meters high, which is rather inconvenient for making (and reading) a barometer. Because mercury () is about -times denser than water, a mercury barometer only needs to be as tall as a water barometer—a more suitable size. The standard atmospheric pressure of 1 atm at sea level () corresponds to a column of mercury that is about ( in.) high. The was originally intended to be a unit equal to one millimeter of mercury, but it no longer corresponds exactly. The pressure exerted by a fluid due to gravity is known as hydrostatic pressure, :
where is the height of the fluid, (lowercase Greek letter rho) is the density of the fluid, and is acceleration due to gravity.